Have you ever wondered what gives laughing gas its unique properties? The answer lies in its molecular structure and, importantly, its molar mass. As a chemist, I’m fascinated by the interplay between molecular weight and chemical behavior. N2O, or nitrous oxide, is a great example of this principle in action. It’s a colorless, odorless gas that’s used in dentistry to alleviate pain, but it’s also a potent greenhouse gas. Understanding the molar mass of nitrous oxide helps us explain these diverse applications and environmental impacts.
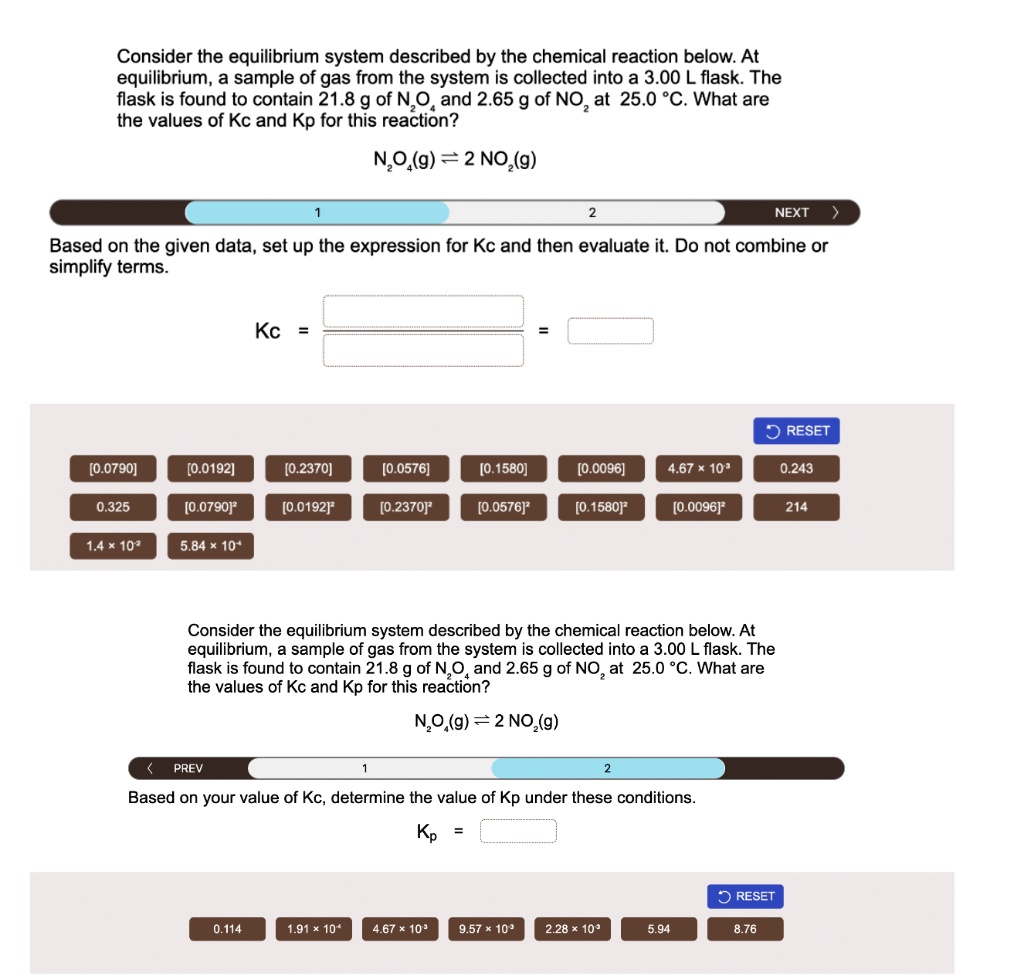
Image: www.numerade.com
In this article, we’ll delve into the world of N2O molar mass. We’ll explore its definition, significance, and real-world applications. We’ll also discuss the latest trends and developments related to this intriguing compound.
Understanding N2O Molar Mass: A Foundation for Chemistry
Defining Molar Mass
Molar mass, simply put, is the mass of one mole of a substance. A mole is a unit of measurement in chemistry that represents a specific number of particles, specifically 6.022 x 1023 particles. This vast quantity is referred to as Avogadro’s number. So, the molar mass of a substance tells us the mass of that substance when we have 6.022 x 1023 of its molecules or atoms.
The molar mass is expressed in grams per mole (g/mol). For example, the molar mass of water, H2O, is 18.015 g/mol. This means that one mole of water weighs 18.015 grams.
Calculating the Molar Mass of N2O
To determine the molar mass of any compound, including N2O, we need to consider the atomic masses of its constituent elements. The periodic table is our go-to resource for this information. Here’s how to calculate the molar mass of N2O:
- Nitrogen (N): The atomic mass of nitrogen is 14.007 g/mol. Since N2O has two nitrogen atoms, we multiply this value by 2: 14.007 g/mol x 2 = 28.014 g/mol
- Oxygen (O): The atomic mass of oxygen is 15.999 g/mol.
- Total Molar Mass: Add the contributions from nitrogen and oxygen: 28.014 g/mol + 15.999 g/mol = 44.013 g/mol
Therefore, the molar mass of N2O, or nitrous oxide, is 44.013 g/mol.

Image: www.youtube.com
Applications of the Molar Mass of N2O
The molar mass of N2O has a significant impact on its properties and applications. Its relatively low molecular weight contributes to its volatility – it easily turns into a gas at room temperature. This volatility makes it suitable for use as an anesthetic, a pain reliever, and a propellant in whipping cream dispensers.
However, the low molecular weight of N2O also has environmental implications. N2O is a potent greenhouse gas, meaning it traps heat in the atmosphere and contributes to global warming. Its low molecular weight allows it to persist in the atmosphere for a long time, exacerbating its effect on climate change.
The Future of N2O: Balancing Utility and Environment
The use of N2O is facing increasing scrutiny due to its environmental impact. Research is ongoing to develop alternative anesthetic agents and propellants that are more environmentally friendly. One promising area is the exploration of alternative gases, such as xenon, which have similar anesthetic properties but are less harmful to the atmosphere.
There’s also a growing focus on reducing emissions of N2O from industrial processes. Many industries use nitrous oxide as a byproduct, and efforts are being made to capture and reuse these emissions. This includes using N2O as a feedstock for other chemical reactions, minimizing its release into the atmosphere.
Tips for Understanding Molar Mass
Understanding molar mass is a cornerstone of chemistry. Here are a few tips to help you grasp this fundamental concept:
- Practice, practice, practice! Calculate the molar mass of different compounds and elements to solidify your understanding.
- Visualize the concept. Imagine a scale with a mole of a substance on one side and its corresponding mass on the other. This mental image can help connect the abstract concept of a mole to a tangible quantity.
- Don’t be afraid to ask for help. If you’re struggling with a specific calculation, seek guidance from your teacher, professor, or a tutor.
Remember, understanding molar mass is about more than just the numbers. It’s about understanding the relationship between mass, atoms, and molecules. This understanding is fundamental to a deeper appreciation of the chemical world around us.
FAQs about N2O and Molar Mass
What is the chemical formula of laughing gas?
The chemical formula for laughing gas, or nitrous oxide, is N2O.
Why is the molar mass of N2O important?
The molar mass of N2O is important because it directly influences its properties and applications. Its low molecular weight makes it suitable for use as an anesthetic but also contributes to its environmental impact as a greenhouse gas.
How does the molar mass of N2O affect its use as an anesthetic?
The low molecular weight of N2O allows it to easily diffuse into and out of the body, making it a suitable anesthetic. It also has a rapid onset of action and short duration, making it ideal for short procedures.
What are some environmental concerns related to N2O?
N2O is a potent greenhouse gas with a long atmospheric lifetime. Its emissions contribute to climate change and atmospheric ozone depletion.
N2o Molar Mass
Conclusion
The molar mass of N2O, 44.013 g/mol, is a crucial factor in understanding the properties and applications of this fascinating compound. It explains its volatility as an anesthetic, but also highlights its role as a powerful greenhouse gas. As we move towards a more sustainable future, research and innovation are crucial for mitigating the environmental impact of N2O while harnessing its beneficial properties.
Are you interested in learning more about N2O and its applications, or perhaps exploring other interesting concepts in chemistry? Let us know in the comments below!

:max_bytes(150000):strip_icc()/OrangeGloEverydayHardwoodFloorCleaner22oz-5a95a4dd04d1cf0037cbd59c.jpeg?w=740&resize=740,414&ssl=1)




