The world of chemistry can feel like a vast, mysterious landscape. We often take for granted the things we see and interact with, but beneath the surface lies a complex and fascinating realm of atoms and molecules. At the heart of this realm lies the concept of orbitals, which dictate the behavior and interactions of electrons within an atom. From the humble water molecule to the intricate macromolecules of life, orbitals play a crucial role in shaping our universe. Today, we delve into the intriguing world of the 3p orbital, exploring its structure, properties, and significance in the realm of atomic physics.
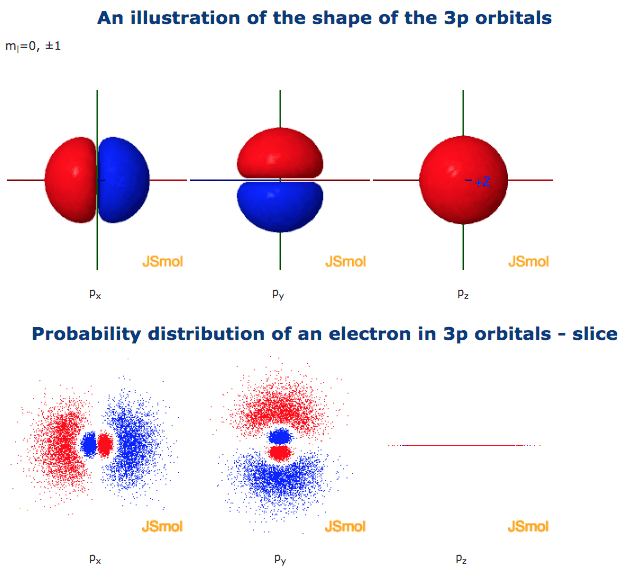
Image: www.chemtube3d.com
My own journey into the world of orbitals began during a high school chemistry class. Our teacher, Mr. Johnson, used a simple analogy to explain the concept of electron configurations. He described the atom as a miniature solar system, with the nucleus at the center and electrons orbiting around it in specific energy levels. As a budding young scientist, I was captivated by this imagery, and it sparked my curiosity to learn more about the intricacies of atomic structure. This interest led me to explore the fascinating world of orbitals, and I’ve been exploring their secrets ever since.
Unveiling the Structure and Properties of the 3p Orbital
The 3p orbital is a type of atomic orbital that resides in the third principal energy level (n = 3) of an atom. Unlike the spherical s orbitals, the p orbitals are shaped like dumbbells, with two lobes oriented along the x, y, or z axes. The 3p orbital, in particular, has a specific spatial orientation that influences its interactions with other orbitals.
The 3p orbital possesses a unique set of properties that contribute to its significance in chemistry. First, its shape and orientation give rise to specific directional bonds, influencing the geometry of molecules. Second, the 3p orbital’s energy level determines its role in chemical reactions, influencing the types of bonds it can form. Finally, the 3p orbital’s capacity to hold two electrons with opposite spins is a fundamental principle governing chemical bonding and reactivity.
Delving Deeper into the 3p Orbital
To understand the 3p orbital’s importance, we need to delve deeper into its characteristics. The 3p orbital, like all p orbitals, has a node, a region where the probability of finding an electron is zero. This node is located at the nucleus, separating the two lobes of the dumbbell-shaped orbital. This nodal structure is crucial in determining the orbital’s spatial properties and its interactions with other orbitals.
Furthermore, the 3p orbital has a specific angular momentum, denoted by the quantum number l = 1. This angular momentum dictates the shape and orientation of the orbital, defining its dumbbell-like form and its alignment along the x, y, or z axes. The 3p orbital, therefore, can exist in three distinct spatial orientations, each labeled as 3px, 3py, and 3pz, corresponding to its orientation along the x, y, and z axes respectively. These orientations play a crucial role in determining the directionality of chemical bonds.
The energy level of the 3p orbital is also critical in understanding its behavior. The higher the energy level, the farther the electron is from the nucleus and the more likely it is to participate in chemical reactions. The 3p orbital resides in the third energy level, implying it’s higher in energy than the 2p orbitals but lower than the 4p orbitals. This energy level determines the orbital’s reactivity and its role in chemical bonding.
Finally, the 3p orbital, like all atomic orbitals, can hold a maximum of two electrons with opposite spins. This principle, known as the Pauli exclusion principle, dictates that no two electrons in an atom can have the same set of quantum numbers. This concept is crucial in understanding the formation of chemical bonds, where electrons from different atoms pair up to form a stable molecule.
Beyond the Basics: Latest Trends and Developments
Research on atomic orbitals continues to evolve, with cutting-edge advancements in computational chemistry and spectroscopy providing deeper insights into their behavior. One exciting development is the use of advanced computational methods to simulate the complex electronic structures of molecules, offering a more detailed understanding of orbital interactions and their influence on chemical reactivity. This allows scientists to predict the properties of molecules with greater accuracy, paving the way for the design of new materials and pharmaceuticals.
Another significant trend is the ongoing quest to visualize and manipulate orbitals using advanced microscopy techniques. By utilizing high-resolution imaging methods, scientists are gaining unprecedented insights into the spatial distribution of electrons within atoms and molecules. This ability to visualize orbitals opens up new possibilities for understanding and influencing chemical reactions at the nanoscale, potentially leading to the development of novel catalysts and materials with tailored properties.
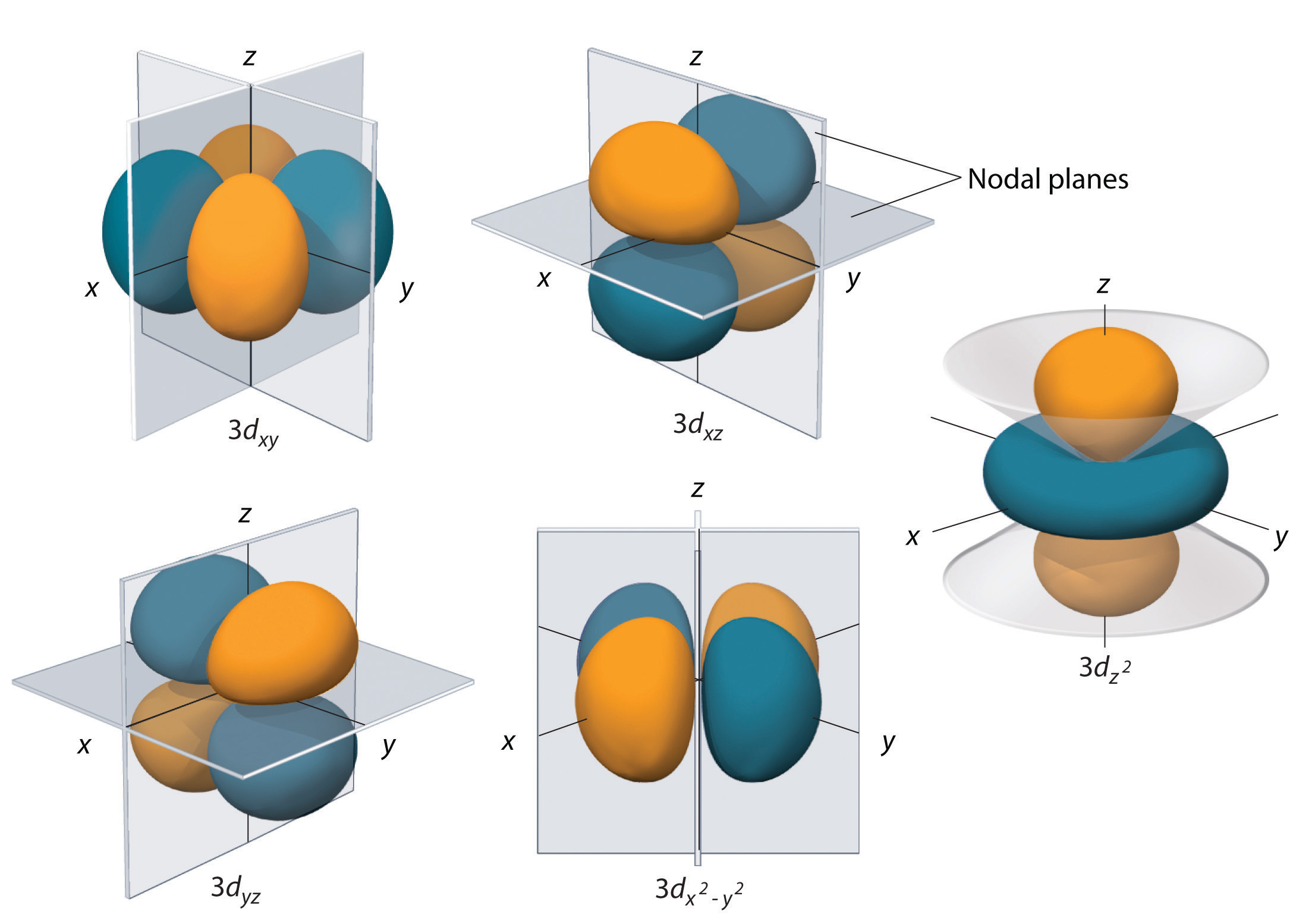
Image: socratic.org
Expert Advice for Exploring the 3p Orbital
I’ve learned that understanding the 3p orbital is a journey of exploration, and here are a few tips that have helped me along the way:
1. **Embrace Visual Representations:** Visualizing the 3p orbital as a dumbbell-shaped cloud helps grasp its spatial orientation and how it interacts with other orbitals. There are numerous online resources and textbooks featuring visualizations that can enhance your understanding.
2. **Connect the Concepts:** Drawing connections between the 3p orbital’s properties (shape, energy level, nodal structure) and its role in chemical bonding can deepen your comprehension. By understanding how these properties influence chemical reactions, you’ll gain a more holistic perspective.
3. **Seek Real-world Examples:** Explore examples of molecules where the 3p orbital plays a significant role. For instance, consider the bonding in water (H₂O), where the oxygen atom utilizes its 3p orbitals to form covalent bonds with two hydrogen atoms.
Frequently Asked Questions
Q: What is the difference between a 3p orbital and a 2p orbital?
A: The 3p and 2p orbitals are both p orbitals, meaning they have a dumbbell shape and a nodal structure. However, the 3p orbital resides in the third principal energy level, while the 2p orbital occupies the second energy level. This difference in energy level results in the 3p orbital being larger and having a higher energy than the 2p orbital.
Q: How does the 3p orbital influence the properties of atoms and molecules?
A: The 3p orbital’s shape, orientation, and energy level influence the properties of atoms and molecules. Its dumbbell shape and orientation contribute to specific directional bonds, influencing the geometry of molecules. Its energy level determines its role in chemical reactions, affecting the types of bonds it can form. Finally, its ability to hold two electrons with opposite spins is fundamental to chemical bonding and reactivity.
Q: What are some examples of molecules where the 3p orbital plays a crucial role?
A: The 3p orbital plays a crucial role in numerous molecules, including water (H2O), carbon dioxide (CO2), and ammonia (NH3). These molecules all exhibit unique properties influenced by the interactions of the 3p orbitals within their structures.
Q: How can I learn more about the 3p orbital and other atomic orbitals?
A: There are many resources available to deepen your understanding of atomic orbitals. Explore introductory chemistry textbooks, reputable online resources, and videos on platforms like YouTube. You can also join online communities and forums dedicated to chemistry for further discussion and insights.
3p Orbital
Conclusion
The 3p orbital, with its distinct shape, orientation, and energy level, is a fundamental building block of atomic structure. Understanding its properties and role in chemical bonding is essential for unraveling the intricacies of chemical reactions and the behavior of matter. Through exploration, visualization, and real-world examples, we can unlock the mysteries of the 3p orbital and its profound impact on our world.
Are you interested in learning more about the 3p orbital and its role in the world of chemistry? Share your thoughts and questions in the comments section below!

:max_bytes(150000):strip_icc()/OrangeGloEverydayHardwoodFloorCleaner22oz-5a95a4dd04d1cf0037cbd59c.jpeg?w=740&resize=740,414&ssl=1)




