Have you ever wondered what makes your favorite soft drinks fizz or helps create the perfect fluffy cake? The answer might lie in a seemingly simple compound: monosodium phosphate, or NaH2PO4. This unassuming chemical plays vital roles in various industries, from food and beverage production to agriculture and pharmaceuticals. This article delves into the fascinating world of NaH2PO4, exploring its properties, uses, and significance in our daily lives.

Image: www.toppr.com
NaH2PO4, also known as monobasic sodium phosphate, is an inorganic compound that exists as a white, odorless, crystalline solid. It is readily soluble in water and is commonly used as a food additive, a buffering agent, and a component of various industrial processes. Understanding its unique characteristics and diverse applications is essential for appreciating its crucial role in our modern world.
Delving into the Chemistry of NaH2PO4
NaH2PO4 is a salt formed by the reaction of phosphoric acid (H3PO4) with sodium hydroxide (NaOH). The resulting compound contains one sodium cation (Na+) and one dihydrogen phosphate anion (H2PO4-), hence the name “monosodium phosphate.” The chemical formula NaH2PO4 clearly indicates the presence of one sodium atom, one hydrogen atom, two oxygen atoms, and one phosphorus atom.
The unique properties of NaH2PO4 are directly linked to its chemical structure and the nature of the dihydrogen phosphate ion. The hydrogen atoms attached to the phosphate group are readily dissociated, making the compound acidic in nature. However, the presence of the sodium cation partially neutralizes this acidity, resulting in a relatively mild pH for aqueous solutions.
Unveiling the Diverse Applications of NaH2PO4
The versatility of NaH2PO4 is truly impressive. Its ability to act as a buffer, an emulsifier, a food additive, and an ingredient in various industrial processes has led to its widespread use in numerous sectors.
1. NaH2PO4 in the Food Industry:
NaH2PO4 is a common food additive due to its ability to act as a buffer, controlling the pH of food products. This is particularly important for maintaining the stability and flavor of many baked goods, beverages, and processed foods. For instance, NaH2PO4 helps stabilize the pH of soft drinks, preventing them from becoming too acidic and affecting their taste. In baking, it acts as a leavening agent, reacting with baking soda to produce carbon dioxide, resulting in a light and fluffy texture.
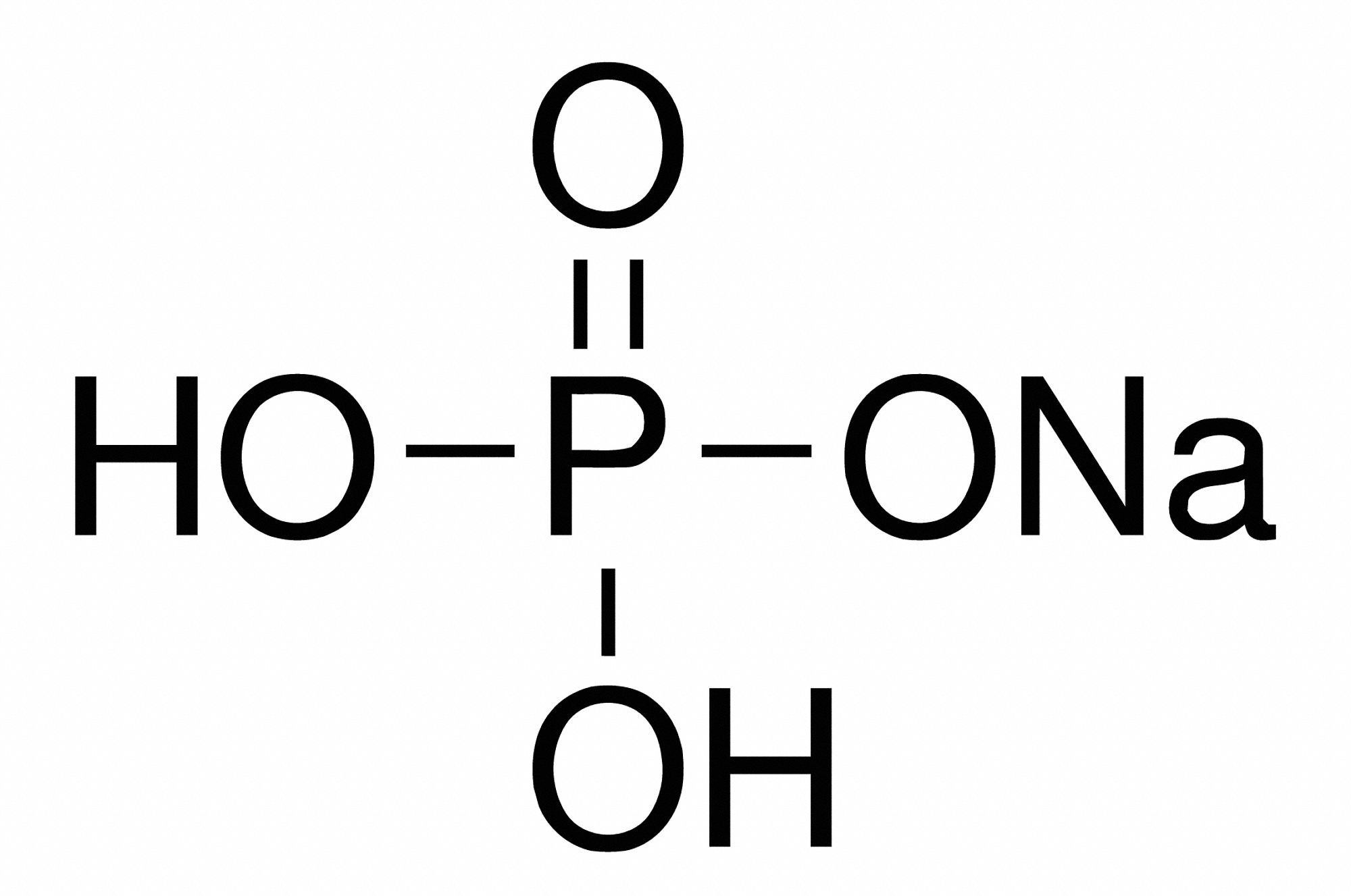
Image: www.grainger.com
2. NaH2PO4 in the Agricultural Sector:
In agriculture, NaH2PO4 plays a crucial role as a phosphate source for plants. Phosphate is an essential nutrient for healthy plant growth and development, and NaH2PO4 provides a readily available form of phosphate that plants can easily absorb. It is often incorporated into fertilizers to enhance soil fertility and promote plant production.
3. NaH2PO4 in Pharmaceuticals and Other Industries:
NaH2PO4 has applications in various industries, including pharmaceuticals, textiles, and metal processing. In pharmaceutical production, it acts as a buffer in oral and injectable medications, maintaining the stability of the solutions. In textile dyeing and finishing, it helps control the pH of the dye bath, ensuring proper dye penetration and colorfastness.
4. NaH2PO4 as a Water Softener:
The ability of NaH2PO4 to bind to calcium and magnesium ions makes it an effective water softener. Hard water, rich in these minerals, can clog pipes and appliances, leading to decreased efficiency and higher energy consumption. NaH2PO4 can remove these ions, softening the water and improving its quality.
Environmental Considerations and Safety of NaH2PO4
While NaH2PO4 is generally considered safe for human consumption in moderate amounts, it is essential to be aware of its potential environmental impacts. Large-scale industrial usage of NaH2PO4 can lead to water pollution if not managed properly, as excessive phosphate levels can cause algal blooms and disrupt aquatic ecosystems.
Further Research and Future Trends
As research continues, the potential applications of NaH2PO4 are constantly being explored. Current research focuses on developing new and sustainable methods for its production and utilization. Exploring ways to reduce its environmental impact and optimize its use in various industries remains a crucial area of focus.
Nah2po4
Conclusion: The Many Facets of NaH2PO4
From stabilizing your favorite soda to supporting healthy plant growth, NaH2PO4 plays a vital role in our daily lives. Its unique properties and diverse applications make it a truly remarkable chemical compound. Understanding its basic chemistry, diverse uses, and environmental considerations is crucial for appreciating its importance in our modern world. As we delve deeper into the world of NaH2PO4, we may discover even more exciting applications and innovations that improve our lives and contribute to a more sustainable future.

:max_bytes(150000):strip_icc()/OrangeGloEverydayHardwoodFloorCleaner22oz-5a95a4dd04d1cf0037cbd59c.jpeg?w=740&resize=740,414&ssl=1)




