Imagine a world without smartphones, computers, or even the simple act of breathing. It’s hard to fathom, isn’t it? But it’s a testament to the power and importance of the elements, the building blocks of everything around us. And what better guide to these fundamental building blocks than the periodic table, a beautifully organized chart that tells the story of chemistry in its entirety?

Image: www.sciencephoto.com
This seemingly simple grid, with its neatly arranged rows and columns, holds within its squares the secrets of the universe. From the tiniest atoms to the vastness of space, the periodic table reveals the intricate relationships between elements, their properties, and their interactions. Today, we’re going to delve deeper into this fascinating visual tool, exploring the labels that bring meaning and clarity to the elements themselves.
Understanding the Structure: Rows and Columns
Periods: Horizontal Layers of Secrets
The periodic table is organized into horizontal rows known as periods. Each period represents a progressive increase in the number of electron shells surrounding the atom’s nucleus. Elements within the same period share similar properties like reactivity, but their atomic size increases as you move from left to right across the period.
Groups: Vertical Columns of Similarities
Now, let’s move vertically. The periodic table is also arranged into columns called groups. Elements within the same group share the same number of valence electrons, which are the electrons in the outermost shell. These valence electrons determine an element’s chemical behavior and how it interacts with other elements. The elements in a group tend to form similar chemical compounds and show predictable reactivity patterns.
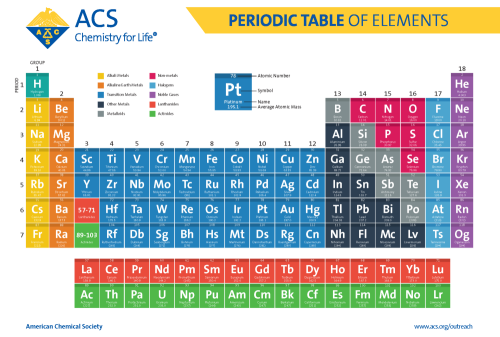
Image: www.kimberlingcity.org
Deciphering the Labels: Essential Information
Atomic Number: Unveiling the Identity
The most fundamental label in the periodic table is the atomic number, a unique identifier of each element. It represents the number of protons in the atom’s nucleus. Protons are positively charged particles that, along with neutrons, contribute to an atom’s mass. Since the number of protons defines an element, the atomic number is the key to understanding its identity.
Element Symbol: A Shorthand Notation
To simplify things, each element is represented by a unique symbol, usually derived from its Latin name. For example, hydrogen is represented by “H”, oxygen by “O”, and gold by “Au” (from the Latin “aurum”). This standardized system of symbols is crucial for communication within the scientific community.
Atomic Mass: Weighting the Atoms
The average atomic mass of an element, typically displayed below the element symbol, is another crucial piece of information. It represents the average mass of all the isotopes of that element, taking into account their relative abundances. Isotopes are atoms of the same element but with different numbers of neutrons, thus varying in their atomic mass.
Electron Configuration: Mapping the Electron Orbitals
Delving deeper into the essence of an element, we encounter its electron configuration. This intricate arrangement of electrons within various energy levels and shells determines the element’s characteristics and reactivity. The electron configuration is a shorthand notation that reflects the distribution of electrons around the atom’s nucleus.
Navigating the Table: Elements in Focus
Alkali Metals: Reactive and Metallic
The alkali metals, found in Group 1 of the periodic table, are known for their extreme reactivity. Lithium (Li), sodium (Na), and potassium (K), for instance, readily react with water, often with a vigorous release of energy. Their soft, silvery appearance and high electrical conductivity are typical of metals, making them essential ingredients in batteries and other technological applications.
Alkaline Earth Metals: Slightly Less Reactive
Moving to Group 2, we encounter the alkaline earth metals. These are less reactive than alkali metals but still exhibit metallic properties. Calcium (Ca), magnesium (Mg), and strontium (Sr) play important roles in biological processes, contributing to the strength of bones and the functioning of nerves and muscles.
Halogens: Nonmetals with a Bite
In Group 17, we find the halogens, a diverse group of nonmetals known for their high reactivity. Chlorine (Cl), bromine (Br), and iodine (I) are essential components of various compounds, including table salt and disinfectants. The halogens are renowned for their ability to form anions, negatively charged ions, and their tendency to react with metals to form salts.
Noble Gases: Indifferent and Unreactive
Finally, we reach Group 18, where the noble gases reside. These elements, including helium (He), neon (Ne), and argon (Ar), are known for their inert nature. Their full outer electron shells make them exceptionally stable and resistant to chemical reactions. Although once considered unreactive, noble gases have found applications in lighting, lasers, and even in medicine.
Transition Metals: A World of Colors and Properties
The transition metals, located in the central block of the periodic table, stand out for their diverse properties and applications. From the vibrant colors of copper (Cu) and gold (Au) to the strength of iron (Fe) and titanium (Ti), these elements have shaped human civilization throughout history. Their ability to form various oxidation states and their remarkable catalytic properties make them indispensable in numerous industries.
Beyond the Basics: Applications of the Periodic Table
The periodic table isn’t just a static chart in a textbook; it’s a dynamic tool used across countless fields. From chemistry labs to engineering firms, from medicine to agriculture, the periodic table provides a framework for understanding the world around us and for developing new technologies.
Medicine and Healthcare: Life-Saving Elements
The periodic table is a vital resource in medicine and healthcare. Elements like iodine (I) are crucial for thyroid hormone production, while iron (Fe) is essential for oxygen transport in red blood cells. Calcium (Ca) strengthens bones and teeth, while lithium (Li) is used in the treatment of certain mental health conditions. The periodic table’s insights are essential to developing new drugs, diagnostic tools, and medical interventions.
Environmental Science: Understanding Pollution and Sustainability
In environmental science, the periodic table helps us understand and address environmental challenges such as pollution. Mercury (Hg) and lead (Pb) are heavy metals with known toxic effects on human health and the environment. Understanding their properties and interactions with other elements allows us to develop strategies to reduce their impact on the planet. The periodic table also plays a role in developing renewable energy technologies and sustainable materials, helping us move towards a more sustainable future.
The Ever-Evolving Periodic Table
The periodic table is not static; it continues to evolve as scientists discover new elements and explore their unique properties. In recent decades, new elements have been synthesized in laboratories, expanding our understanding of the universe’s building blocks. The periodic table represents a collective human endeavor to unravel the fundamental secrets of matter, constantly pushing boundaries and shaping our understanding of the world around us.
Periodic Table With Labels
Conclusion
The periodic table with its labels is a powerful tool that unlocks a world of knowledge about the elements, their properties, and their interactions. From understanding the basic building blocks of matter to developing life-saving medicines and tackling environmental challenges, the periodic table continues to be a cornerstone of scientific discovery and innovation. As we continue to explore the universe and unravel its mysteries, the periodic table will undoubtedly remain a vital guide, illuminating the path toward a brighter future.

:max_bytes(150000):strip_icc()/OrangeGloEverydayHardwoodFloorCleaner22oz-5a95a4dd04d1cf0037cbd59c.jpeg?w=740&resize=740,414&ssl=1)




